A research group led by Prof. Wang Honglin at The First People's Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, has developed a drug candidate named CKBA from the active small molecules of frankincense, a traditional Chinese medicine. CKBA binds to microglial MFE-2 (peroxisomal enoyl-CoA hydratase/3-hydroxyacyl-CoA dehydrogenase), preserves peroxisomal lipid metabolism, suppresses chronic central nervous system inflammation and slows Alzheimer’s pathology. The compound readily crosses the blood–brain barrier and shows an excellent safety profile, highlighting strong translational potential.
On 29 October 2025, the team published the findings in Nature Aging under the title “Loss of MFE-2 impairs microglial lipid homeostasis and drives neuroinflammation in Alzheimer’s pathogenesis”. The study shows that down-regulation of MFE-2 in microglia causes lipid accumulation and neuroinflammation, aggravates β-amyloid (Aβ) deposition and amplifies central inflammatory activation. The researchers identified a small molecule that binds MFE-2 with high affinity, restores fatty-acid metabolic balance and thereby suppresses neuroinflammation, providing a new therapeutic target for Alzheimer’s disease.
Research Background
Alzheimer’s disease (AD) is the most prevalent neurodegenerative disorder worldwide, accounting for more than half of all dementia cases and characterised by progressive cognitive decline, widespread neuronal loss and sustained neuroinflammation. Despite intensive research on Aβ and Tau, the core pathogenic mechanisms remain controversial, and the crosstalk between disrupted lipid metabolism and neuroinflammation is poorly understood. Effective molecular interventions are lacking.
The team now offers a fresh route: they reveal for the first time that the peroxisomal β-oxidation enzyme MFE-2 is a metabolic checkpoint for microglial immune homeostasis, define its pivotal position in the AD “lipid–inflammation” axis, and optimise the small-molecule ligand CKBA that exhibits high blood–brain-barrier permeability. In animal models CKBA markedly improves cognition and confers neuroprotection, opening a new avenue for targeted AD therapy. The work appeared in Nature Aging on 29 October 2025 and a patent covering the indication has been filed simultaneously.
Key Results
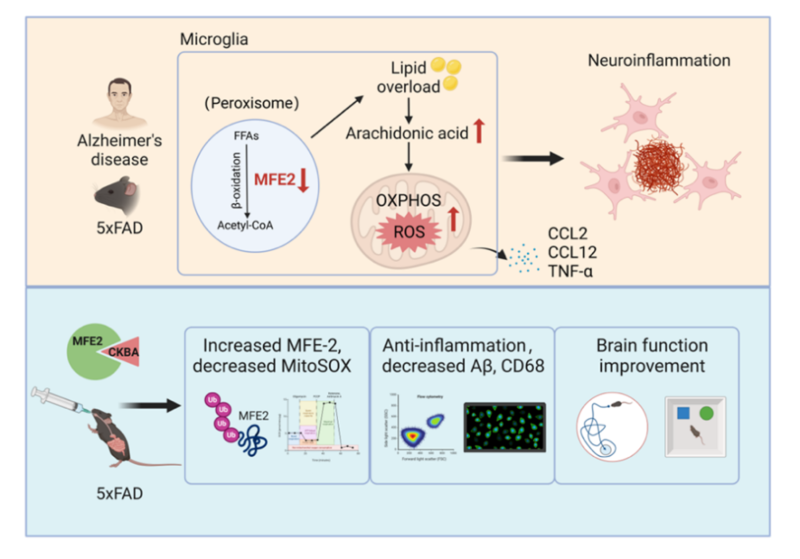
Peroxisomes are indispensable for fatty-acid β-oxidation, especially very-long-chain fatty acids, and are increasingly recognised as regulators of immune-cell homeostasis. The study establishes peroxisomal MFE-2 as a druggable target for AD and screens the natural-product derivative CKBA (from acetyl-11-keto-β-boswellic acid, AKBA). CKBA binds full-length MFE-2 with nanomolar affinity (K<sub>D</sub> = 0.97 nM), blocks inflammation-induced ubiquitination and degradation of the enzyme, and thereby restores microglial lipid homeostasis. Oral administration efficiently delivers CKBA to the brain without overt toxicity, shows favourable pharmacokinetics, and in AD transgenic mice improved memory performance, lowered neuroinflammation, reduced Aβ plaque load and alleviated anxiety-like behaviour (Figure 1).
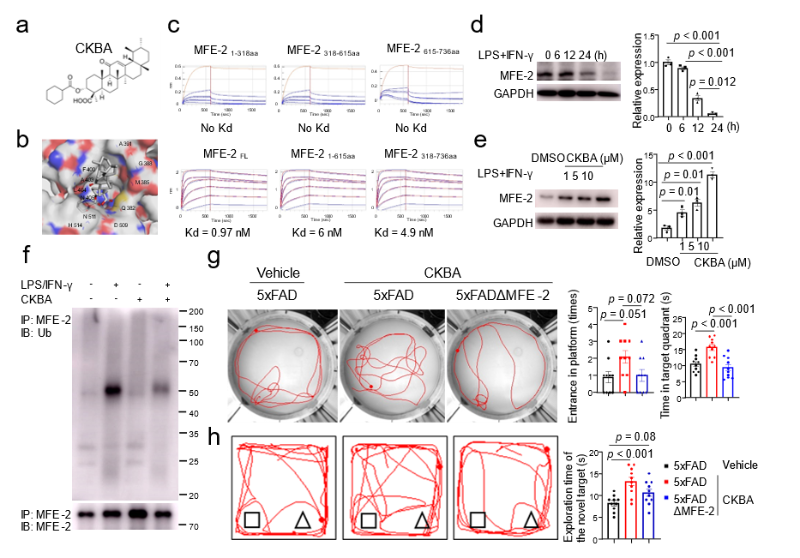
Figure 1. Targeting MFE-2 by CKBA mitigates the progression of Alzheimer's disease pathology
The team further generated microglia-specific MFE-2 knockout AD mice. Loss of MFE-2 exacerbated Aβ deposition, precipitated cognitive decline, shrank microglial processes and elevated global inflammatory tone. Behavioural tests revealed aggravated memory deficits and impaired exploratory activity (Figure 2).
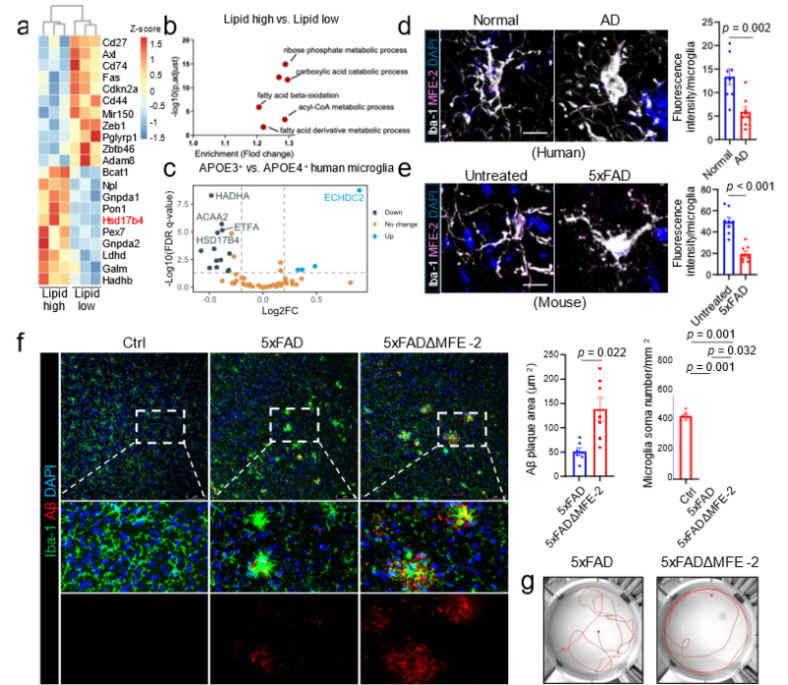
Figure 2. Conditional knockout of MFE-2 exacerbates AD memory deficits by enhancing microglial activation.
Mechanistically, multi-level evidence delineates a coherent pathway: MFE-2 deletion → lipid dysregulation → arachidonic acid accumulation → mitochondrial stress → pro-inflammatory activation → neurodegeneration (Figure 3). The study thus decodes MFE-2 as a metabolic guardian of microglia and validates its therapeutic relevance.
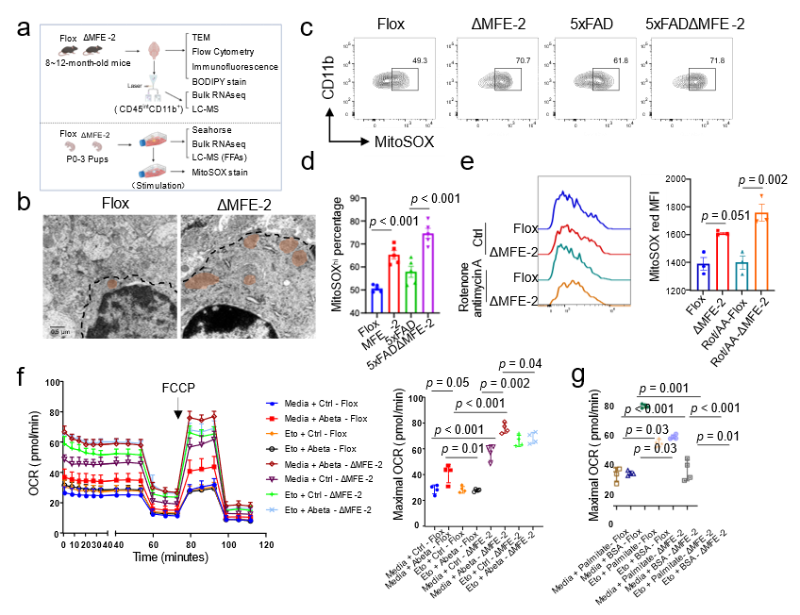
Figure 3. MFE-2 knockout leads to elevated mitochondrial oxidative stress in microglial cells.
In summary, the work uncovers a lipid-metabolic driver of AD neuroinflammation, clarifies the central role of peroxisomal MFE-2 in microglial metabolic stability and delivers CKBA as a first-in-class small-molecule candidate that rescues MFE-2 function and ameliorates neuropathology. The findings introduce the concept of an early “metabolism–inflammation coupling pathway” in Alzheimer’s disease and provide a translational strategy for intervention.
Research Team and Funding Support
The study was led by Prof. Wang Honglin’s group at The First People's Hospital Affiliated to Shanghai Jiao Tong University School of Medicine. The team has long focused on autoimmune disease mechanisms, target discovery and drug development, with recent emphasis on immune-metabolic networks and small-molecule intervention. They have established multiple neurological-disease models and developed a series of brain-penetrant neuroprotective candidates. The current Nature Aging paper represents a key output of their end-to-end pipeline: mechanism → target validation → lead compound → animal proof-of-concept → translational advancement.

Lead Researcher: Wang HongLin
Professor and doctoral supervisor, National Distinguished Young Scholar (2017), Chief Scientist of the National Key R&D Programme (2020), Deputy Director of the National Key Laboratory of Innovative Immunotherapy, Distinguished Professor and Zhiyuan Honour Programme mentor at Shanghai Jiao Tong University, Executive Vice Dean of the Clinical Research Institute and Director of the Precision Medicine Centre for Difficult Diseases at the First People’s Hospital. He has published 78 papers in leading journals (total IF 665, >5,500 citations) and holds 16 Chinese patents and 4 PCT patents.
The work was supported by the NSFC Original Exploration Programme (82450903), Shanghai “Science and Technology Innovation Action” Laboratory Animal Project (22140903100), NSFC General Programme (82073428), and China Postdoctoral Science Foundation (2021M692114, GZB20230431).
Research Paper link:https://doi.org/10.1038/s43587-025-00976-1
Authors: Prof. Wang Honglin’s team, The First People's Hospital Affiliated to Shanghai Jiao Tong University School of Medicine
Source: Institute of Translational Medicine
Translated by: Rebecca
Proof: Steven